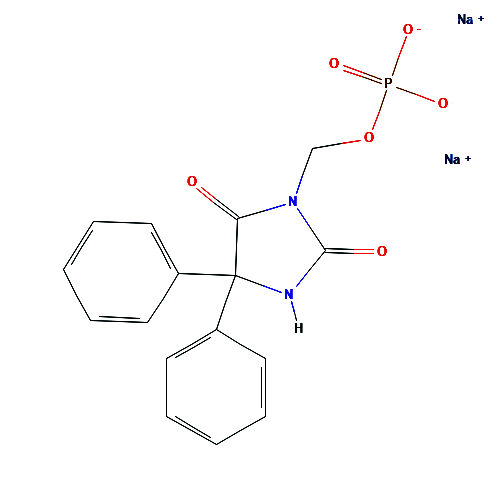
Fosphenytoin Sodium USP
- Product Name : Fosphenytoin Sodium
- CAS : 92134-98-0
- Molecular Formula : C16H13N2Na2O6P
- Molecular Weight : 406.24 g/mol
- Pharmaceutical Grade : USP
- Therapeutic Category : Anticonvulsant, anti-epileptic
Global Calcium is one of the leading manufacturers and exporters of Fosphenytoin Sodium CAS no. 92134-98-0, API, (Active Pharmaceutical Ingredient). We support the customers with exhaustive documentation. As a USDMF & EU-GMP certified global company and an established hallmark for pharmaceutical standards, Global Calcium has stood the test of time since its inception in 1979.
As manufacturer of Fosphenytoin Sodium we hereby state the following facts about the drug:
Fosphenytoin, a phenytoin prodrug, has the same pharmacological properties as phenytoin.
Advantages over phenytoin,
- May be administered by intramuscular injection.
- Lower potential for local tissue and cardiac toxicity than phenytoin.
- Associated with less pain and phlebitis at the injection site, fewer reductions in infusion rate and fewer changes of administration site because of injection site complications than phenytoin.
- Benefits in terms of ease of administration and improved tolerability vs phenytoin.
All other factors being equal, there is no doubt that fosphenytoin is better tolerated and can be delivered faster than intravenous phenytoin. The tolerability of intramuscular fosphenytoin also extends its use to clinical situations where prompt administration of a non-depressing anticonvulsant is indicated but secure intravenous access and cardiac monitoring are not available, such as treatment of seizures by rescue squads in the field and serial seizures in the institutionalised, elderly and other patients with intractable epilepsy.
Global Calcium is a leading manufacturer of this drug. We manufacture this pharmaceutical drug and make it available to domestic and overseas market